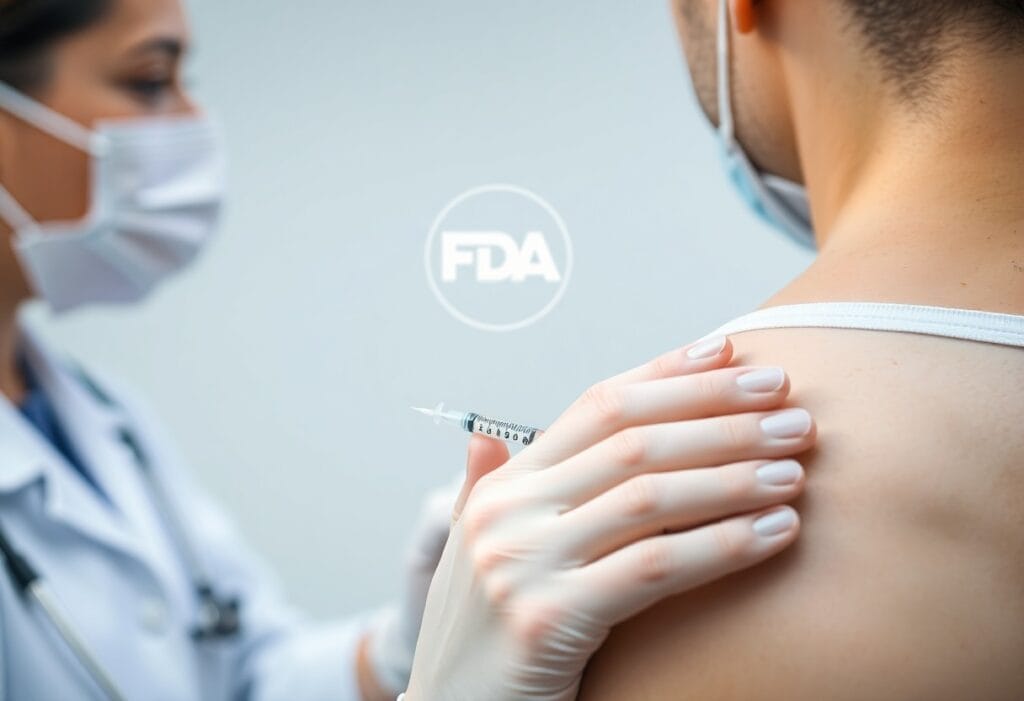
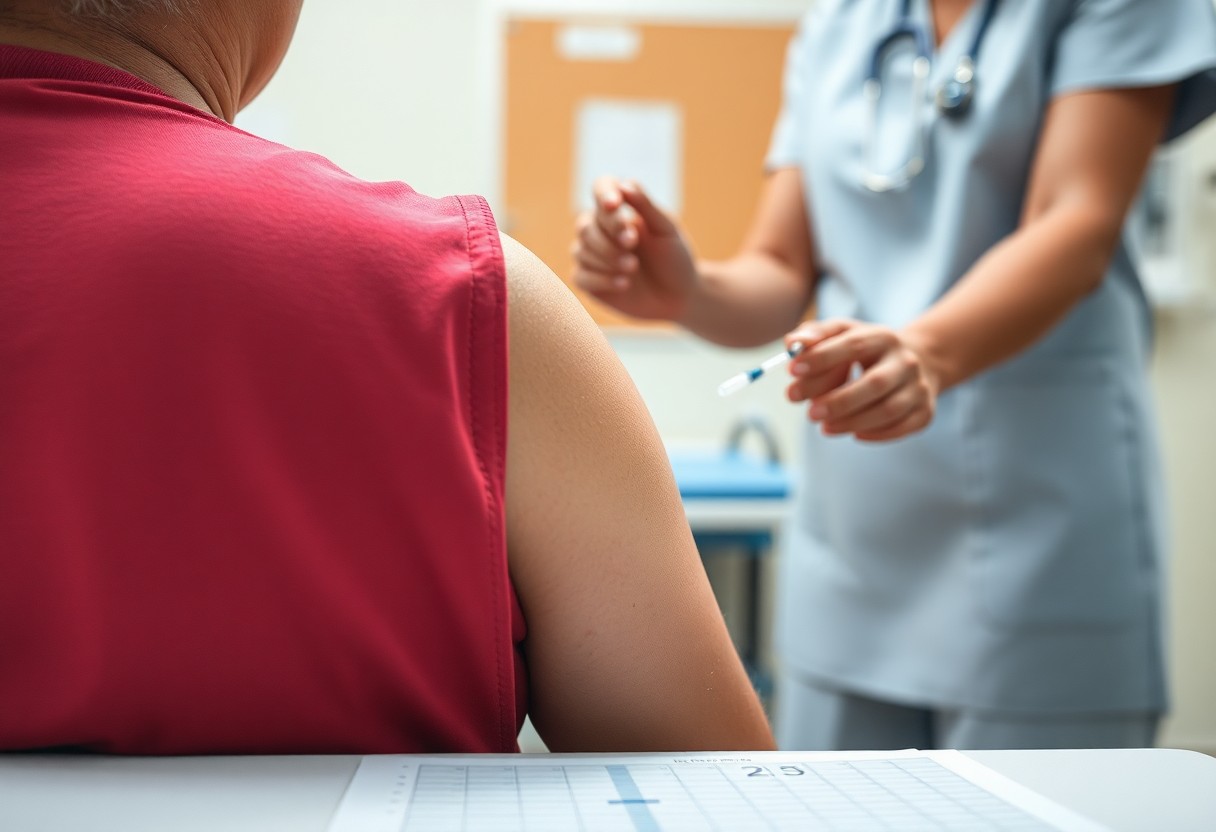
Just to ensure your health and safety, it’s crucial to understand SIRVA injuries, or Shoulder Injury Related to Vaccine Administration. The Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) provide valuable insights on how these injuries can occur during vaccine administration and the preventive measures to take. By being informed, you can protect yourself from potential complications and make knowledgeable decisions about your vaccination process. Learn the details the CDC and FDA recommend to minimize your risk of SIRVA injuries.
Understanding SIRVA
A SIRVA, or Shoulder Injury Related to Vaccine Administration, occurs when you sustain injury to your shoulder following a vaccine injection. This can lead to pain and reduced range of motion, making everyday activities challenging. Incidents of SIRVA typically arise from improper injection techniques that result in injury to the shoulder structures.
Definition of SIRVA
Across the medical community, SIRVA is recognized as a condition that arises due to inappropriate vaccine administration. This includes injections that are given too high on the arm, which may impact shoulder structures and lead to serious discomfort or persistent pain.
Causes and Risk Factors
For you to fully understand SIRVA, it’s imperative to be aware of its causes and risk factors. They can include the following:
- Improper injection site
- Injection technique
- Patient anatomy
- Previous shoulder injuries
Any of these factors can contribute to the likelihood of developing SIRVA.
Understanding the causes and risk factors associated with SIRVA is imperative for prevention. It is important to be aware that a poor injection technique can significantly raise your risk of injury. Ensuring that vaccine administration occurs at the correct site is vital to avoid complications. Additionally, individuals with previous shoulder injuries may want to discuss their history with their healthcare provider before receiving vaccinations, as this can inform better care. Any lack of awareness about these factors can lead to serious consequences for your shoulder health.
CDC Guidelines on SIRVA
While the CDC emphasizes the importance of vaccination, they also highlight the potential risk of Shoulder Injury Related to Vaccine Administration (SIRVA). Understanding how to properly administer vaccines and recognizing the signs of SIRVA can enhance overall patient safety and help you avoid complications from vaccination.
Recommendations for Vaccine Administration
To minimize the risk of SIRVA, the CDC recommends administering vaccines in the deltoid muscle, ensuring the needle is inserted at the correct angle and depth. Additionally, you should avoid injecting into areas of the arm that are compromised by prior injury or anatomical anomalies.
Identifying Symptoms of SIRVA
Behind the scenes, SIRVA symptoms can develop rapidly following vaccination. Common signs include persistent shoulder pain, reduced range of motion, and localized swelling. Early recognition of these symptoms is imperative for effective management and treatment.
Guidelines for identifying symptoms of SIRVA involve awareness of the timing and severity of pain, which typically arises within 48 hours post-vaccination. If you experience ongoing pain that is not improving, or if you notice swelling and decreased motion in your shoulder, it is important to seek medical advice promptly. Prompt identification and intervention can lead to better outcomes and help you avoid long-term complications.

FDA Perspective on SIRVA
If you are concerned about SIRVA, it’s important to understand the FDA’s perspective. The FDA recognizes SIRVA as a potential side effect of vaccinations, particularly those administered in the deltoid muscle. The agency closely monitors vaccine safety and continuously reviews data related to adverse events, including SIRVA, ensuring that health professionals and the public remain informed about potential risks and benefits associated with vaccines.
Reporting and Monitoring SIRVA Cases
After identifying a SIRVA case, healthcare providers have the responsibility to report the event to the Vaccine Adverse Event Reporting System (VAERS). This vital process aids in the ongoing monitoring of vaccine safety. By reporting, you contribute to a comprehensive database that health authorities use to evaluate patterns and ensure that vaccines remain safe for public use.
Safety Protocols for Vaccine Manufacturers
SIRVA is taken seriously, leading the FDA to enforce strict safety protocols for vaccine manufacturers. These protocols are designed to minimize risks associated with vaccine administration, including proper training for healthcare professionals on injection techniques. By promoting correct injection practices and utilizing specific measurement tools, manufacturers aim to reduce the likelihood of SIRVA occurring.
Plus, vaccine manufacturers are required to conduct rigorous testing and post-marketing surveillance to actively identify and address any potential risks. This commitment to safety means that if you experience SIRVA symptoms, your concerns will be taken seriously and investigated thoroughly. Ongoing education about safe injection practices further ensures that patients receive vaccinations with minimal risks, contributing to the overall effectiveness and safety of immunizations.
Treatment Options for SIRVA
Now, addressing SIRVA injuries involves a combination of non-invasive therapies and, in some cases, medical interventions. Your treatment plan will likely begin with conservative measures, such as rest, ice application, and over-the-counter pain relievers. However, if symptoms persist, your healthcare provider may recommend more specialized treatments to ensure a full recovery.
Physical Therapy
Around the world of rehabilitation, physical therapy plays a significant role in treating SIRVA. You may work with a physical therapist to perform exercises aimed at restoring shoulder mobility, improving strength, and reducing pain. Customized therapy can help you regain functionality and prevent future complications.
Medical Interventions
On the medical side, various interventions may be necessary for managing your symptoms. These can include corticosteroid injections to reduce inflammation or pain relief, as well as medications to facilitate recovery. Your healthcare provider will assess your situation to determine the best course of action.
And, in some cases, surgical intervention may be needed if conservative treatments fail. While surgery carries inherent risks, it can also lead to significant improvements in your quality of life and alleviate chronic pain resulting from SIRVA. Discussing all available options with your healthcare provider ensures that you will receive the most suitable treatment plan tailored specifically for your needs.
Prevention Strategies
All individuals can significantly reduce the risk of SIRVA injuries by following key prevention strategies. Engaging in proper injection techniques and ensuring that you take appropriate post-vaccination care are imperative steps in minimizing discomfort and potential long-term complications.
Proper Injection Techniques
On receiving a vaccination, it’s vital that healthcare providers utilize proper injection techniques to avoid SIRVA. This includes selecting the correct site for the injection, using the appropriate needle size, and ensuring proper administration to prevent damage to surrounding tissues.
Post-vaccination Care
Along with proper injection techniques, your post-vaccination care plays a significant role in preventing SIRVA. Following the vaccination, stay alert for any unusual pain or swelling in your upper arm, as taking immediate action can help prevent serious complications.
Plus, it’s beneficial to apply a cold compress to the injection site and avoid strenuous activity for a couple of days. You should maintain good arm movement to promote healing and monitor for any signs of worsening pain or restricted mobility. It’s imperative to seek medical attention if you experience severe pain, excessive swelling, or persistent symptoms that could indicate a SIRVA injury. Prompt action can decrease long-term complications and ensure a quicker recovery.
SIRVA in Context: Vaccination Safety
For anyone considering vaccination, it is important to understand the safety protocols that surround these medical procedures. The CDC and FDA emphasize that vaccines undergo rigorous testing to ensure their efficacy and safety. While SIRVA injuries can occur in rare instances, the overall benefits of vaccination far outweigh the potential risks. Therefore, you can feel confident knowing that vaccination remains a vital tool in protecting your health and the health of your community.
Public Health Implications
Across the globe, vaccination programs are critical in controlling infectious diseases and promoting public health. Understanding the potential for SIRVA injuries helps in developing informed consent processes and safe vaccination practices. Awareness of vaccine-related injuries ensures that healthcare providers can monitor and respond to adverse events effectively, thereby increasing your confidence in vaccination initiatives.
Addressing Vaccine Hesitancy
Along with ensuring safety, addressing vaccine hesitancy is vital in promoting widespread vaccination. Your concerns about vaccines often stem from misinformation or uncertainty regarding potential side effects.
With various sources spreading misleading information, it is crucial to provide you with accurate, evidence-based details about the safety of vaccines. Additionally, positive testimonials from those who have been successfully vaccinated can play a significant role in alleviating your fears. Health experts encourage open dialogues that address your concerns while reinforcing the overwhelming evidence showing that vaccines protect against serious diseases. Ultimately, empowering you with knowledge can combat hesitancy, leading to a healthier community for everyone.
Summing up
Upon reflecting on what the CDC and FDA say about SIRVA injuries, it’s imperative for you to understand that these injuries can occur due to improper vaccine administration, particularly in the shoulder area. Both organizations emphasize the importance of proper injection technique to minimize the risk of SIRVA. You should be aware that if you experience shoulder pain following a vaccination, it’s important to discuss this with your healthcare provider to address potential complications and receive appropriate care. Being informed empowers you to advocate for your health effectively.